NEBNext® Ultra™ II FS DNA Library Prep Kit for Illumina
- 价格: ¥29379/盒
- 发布日期: 2023-07-05
- 更新日期: 2026-01-14
产品详请
| 产地 |
|
| 品牌 |
New England Biolabs
|
| 货号 |
E7805L
|
| 用途 |
|
| 包装规格 |
96T
|
| 纯度 |
%
|
| CAS编号 |
|
| 别名 |
|
| 是否进口 |
是
|
The NEBNext® Ultra™ II FS DNA Library Prep Kit for Illumina provides a fragmentation system: a fast and reliable solution for DNA fragmentation and library construction. A new DNA fragmentation reagent is combined with end repair and dA-tailing reagents, allowing these steps to be performed in the same tube, with no clean-ups or sample loss. The same fragmentation protocol is used for any input amount (100 pg–500 ng), or GC content. The kit also includes the NEBNext Ultra II Ligation Master Mix for adaptor ligation, and the NEBNext Ultra II Q5® Master Mix for uniform, high-fidelity library amplification. Please note that adaptors and primers are not included in the kit and are available separately.
For protocols including bisulfite converted DNA, we recommend the NEBNext Ultra II DNA Library Prep Kit for Illumina .
Features:
-
Fragmentation, end repair and dA-tailing reagents in a single enzyme mix
-
A single fragmentation protocol, regardless of DNA input amount or GC content
-
Input amounts: 100 pg–500 ng
-
Input DNA can be in water, Tris or TE
-
Workflow: ~ 2.5 hours, with < 15 minutes hands-on time
Download extensive performance data in our technical note.
Also available with optional SPRIselect® beads for size selection/clean-up steps
For use with NEBNext Multiplex Oligos for Illumina (Unique Dual Index UMI Adaptors DNA Set 1) (NEB #E7395 ), refer to the Protocols tab for UMI Adaptors-specific guidance.
Figure 1: NEBNext Ultra II FS DNA produces the highest yields, from a range of input amounts
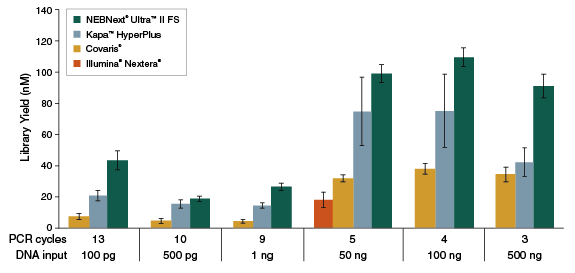 Libraries were prepared from Human NA19240 genomic DNA using the input amounts and numbers of PCR cycles shown. For NEBNext Ultra II FS, a 20-minute fragmentation time was used. For Kapa™ HyperPlus libraries, input DNA was cleaned up with 3X beads prior to library construction, as recommended, and a 20-minute fragmentation time was used. Illumina® recommends 50 ng input for Nextera, and not an input range; therefore, only 50 ng was used in this experiment. “Covaris®” libraries were prepared by shearing each input amount in 1X TE Buffer to an insert size of ~200 bp using a Covaris instrument, followed by library construction using the NEBNext Ultra II DNA Library Prep Kit (NEB #E7645). Error bars indicate standard deviation for an average of 3–6 replicates performed by 2 independent users.Figure 2: NEBNext Ultra II FS DNA provides consistent fragmentation regardless of input amount
Libraries were prepared from Human NA19240 genomic DNA using the input amounts and numbers of PCR cycles shown. For NEBNext Ultra II FS, a 20-minute fragmentation time was used. For Kapa™ HyperPlus libraries, input DNA was cleaned up with 3X beads prior to library construction, as recommended, and a 20-minute fragmentation time was used. Illumina® recommends 50 ng input for Nextera, and not an input range; therefore, only 50 ng was used in this experiment. “Covaris®” libraries were prepared by shearing each input amount in 1X TE Buffer to an insert size of ~200 bp using a Covaris instrument, followed by library construction using the NEBNext Ultra II DNA Library Prep Kit (NEB #E7645). Error bars indicate standard deviation for an average of 3–6 replicates performed by 2 independent users.Figure 2: NEBNext Ultra II FS DNA provides consistent fragmentation regardless of input amount

Libraries were prepared from Human NA19240 genomic DNA using the input amounts shown. NEBNext Ultra II FS libraries were prepared using a 20-minute fragmentation time. For Kapa™ HyperPlus, input DNA was cleaned up with 3X beads prior to library construction, as recommended, and a 20-minute fragmentation time. Library size was assessed using the Agilent® Bioanalyzer®. Low input (1 ng and below) libraries were loaded on the Bioanalyzer without a dilution. High input libraries were loaded with a 1:5 dilution in 0.1X TE.Figure 3: NEBNext Ultra II FS DNA provides uniform GC coverage for microbial genomic DNA over a broad range of GC composition
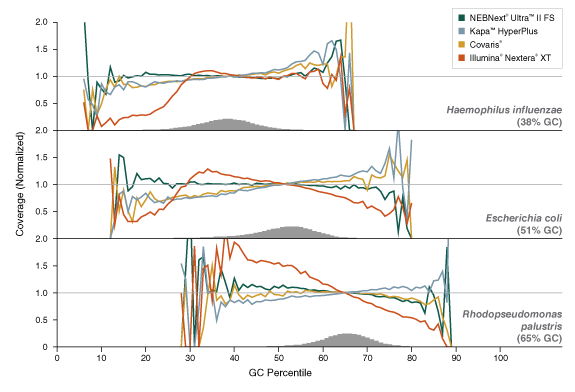 Libraries were prepared using 1 ng of a mix of genomic DNA samples from Haemophilus influenzae, Escherichia coli (K-12 MG1655), Rhodopseudomonas palustris and the library prep kits shown, with 9 PCR cycles for consistency across samples, and sequenced on an Illumina MiSeq®. NEBNext Ultra II FS libraries were prepared using a 20-minute fragmentation time. For Kapa HyperPlus libraries, input DNA was cleaned up with 3X beads prior to library construction, as recommended, followed by a 25-minute fragmentation time. “Covaris” libraries were prepared by shearing 1 ng of DNA in 1X TE Buffer to an insert size of ~200 bp using a Covaris instrument, followed by library construction using the NEBNext Ultra II DNA Library Prep Kit (NEB #E7645). Reads were mapped using Bowtie 2.2.4 and GC coverage information was calculated using Picard’s CollectGCBiasMetrics (v1.117). Expected normalized coverage of 1.0 is indicated by the horizontal grey line, the number of 100 bp regions at each GC% is indicated by the vertical grey bars, and the colored lines represent the normalized coverage for each library.Figure 4: ULTRA II FS produced 10-15x more DNA library compared with other methods - Data from Peter Ellis of the Wellcome Trust Sanger Institute
Libraries were prepared using 1 ng of a mix of genomic DNA samples from Haemophilus influenzae, Escherichia coli (K-12 MG1655), Rhodopseudomonas palustris and the library prep kits shown, with 9 PCR cycles for consistency across samples, and sequenced on an Illumina MiSeq®. NEBNext Ultra II FS libraries were prepared using a 20-minute fragmentation time. For Kapa HyperPlus libraries, input DNA was cleaned up with 3X beads prior to library construction, as recommended, followed by a 25-minute fragmentation time. “Covaris” libraries were prepared by shearing 1 ng of DNA in 1X TE Buffer to an insert size of ~200 bp using a Covaris instrument, followed by library construction using the NEBNext Ultra II DNA Library Prep Kit (NEB #E7645). Reads were mapped using Bowtie 2.2.4 and GC coverage information was calculated using Picard’s CollectGCBiasMetrics (v1.117). Expected normalized coverage of 1.0 is indicated by the horizontal grey line, the number of 100 bp regions at each GC% is indicated by the vertical grey bars, and the colored lines represent the normalized coverage for each library.Figure 4: ULTRA II FS produced 10-15x more DNA library compared with other methods - Data from Peter Ellis of the Wellcome Trust Sanger Institute
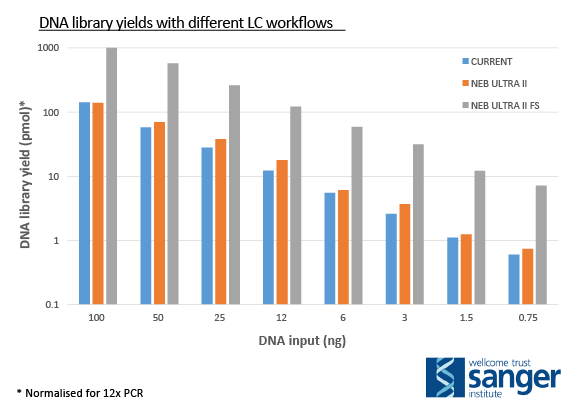 Human genomic DNA was subjected to DNA library construction using an existing DNA library construction workflow (CURRENT), NEB Ultra II or NEB Ultra II FS. Adapter-Ligated libraries were amplified by PCR (4-12 cycles), purified and quantitated using the Agilent Bioanalyzer platform. Values obtained were used to normalize DNA library yield from 12 cycles of PCR.
Human genomic DNA was subjected to DNA library construction using an existing DNA library construction workflow (CURRENT), NEB Ultra II or NEB Ultra II FS. Adapter-Ligated libraries were amplified by PCR (4-12 cycles), purified and quantitated using the Agilent Bioanalyzer platform. Values obtained were used to normalize DNA library yield from 12 cycles of PCR.
View additional data presented at AGBT by Peter Ellis, Senior Staff Scientist at the Wellcome Trust Sanger Institute.





